Page 69 - Energize July 2022
P. 69
TECHNICAL
discharges which occur but there can
be frequent small discharges. In most
cases the loads on the battery are high in
relation to the nominal capacity.
For a long time the central office was
the switching centre for transporting
all voice communication, and VLAs
were the battery of choice. With the
advent of mobile communications and
the digitizing of voice, video and data,
the telecommunications industry uses
a combination of battery types which
includes both the VLA and the VRLA cells
(individual 2 V cells and 12 V monoblocs),
and in some instances even Ni-Cds.
The important common factor in all
of the applications described above is
that these are always maintained and
operated at 100% state-of-charge (SOC).
That being the case, let us now look at
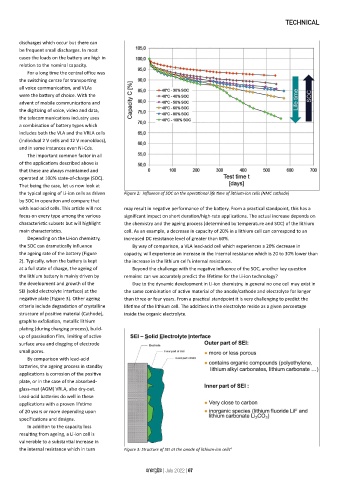
the typical ageing of Li-ion cells as driven Figure 2: Influence of SOC on the operational life time of lithium-ion cells (NMC cathode)
by SOC in operation and compare that
with lead-acid cells. This article will not may result in negative performance of the battery. From a practical standpoint, this has a
focus on every type among the various significant impact on short duration/high-rate applications. The actual increase depends on
characteristic subsets but will highlight the chemistry and the ageing process (determined by temperature and SOC) of the lithium
main characteristics. cell. As an example, a decrease in capacity of 20% in a lithium cell can correspond to an
Depending on the Li-ion chemistry, increased DC resistance level of greater than 60%.
the SOC can dramatically influence By way of comparison, a VLA lead-acid cell which experiences a 20% decrease in
the ageing rate of the battery (Figure capacity, will experience an increase in the internal resistance which is 20 to 30% lower than
2). Typically, when the battery is kept the increase in the lithium cell’s internal resistance.
at a full state of charge, the ageing of Beyond the challenge with the negative influence of the SOC, another key question
the lithium battery is mainly driven by remains: can we accurately predict the lifetime for the Li-ion technology?
the development and growth of the Due to the dynamic development in Li-ion chemistry, in general no one cell may exist in
SEI (solid electrolyte interface) at the the same combination of active material of the anode/cathode and electrolyte for longer
negative plate (Figure 3). Other ageing than three or four years. From a practical standpoint it is very challenging to predict the
criteria include degradation of crystalline lifetime of the lithium cell. The additives in the electrolyte reside as a given percentage
structure of positive material (Cathode), inside the organic electrolyte.
graphite exfoliation, metallic lithium
plating (during charging process), build-
up of passivation film, limiting of active
surface area and clogging of electrode
small pores.
By comparison with lead-acid
batteries, the ageing process in standby
applications is corrosion of the positive
plate, or in the case of the absorbed-
glass-mat (AGM) VRLA, also dry-out.
Lead-acid batteries do well in these
applications with a proven lifetime
of 20 years or more depending upon
specifications and designs.
In addition to the capacity loss
resulting from ageing, a Li-ion cell is
vulnerable to a substantial increase in
the internal resistance which in turn Figure 3: Structure of SEI at the anode of lithium-ion cells
4
energize | July 2022 | 67